Empower Your Clinical Trials Software
with  Business Intelligence and Analytics Platform
Business Intelligence and Analytics Platform

Advertising your product without BI features can be difficult. You need a strategic, experienced BI partner that provides OEM services to ensure hassle-free, cost-effective and lucrative sustainable outcomes.
Enhance your CTMS software with TURBOARD's OEM services to deliver unparalleled analytics capabilities. By integrating our advanced BI tools, you can offer your clients more robust data processing, insightful analytics, and interactive reporting features.
This integration enables CTMS software providers to stand out in the market, offering a more comprehensive solution that not only streamlines clinical trial management but also provides deep insights into trial data, ultimately leading to more informed decisions and successful outcomes. Click here to discover more about TURBOARD's capabilities in the Clinical Research and Trials sector.
Why choose  as your OEM partner?
as your OEM partner?
Our Experience in BI & Data Analytics
|
Our Experience in OEM
|
Adding features and developing analytics capabilities in-house for your clinical trials’ software requires a dedicated team of BI experts, developers, designers, and data scientists, which can be very costly and time-consuming. On the other hand, you may consider partnering with an experienced BI vendor.
We would be happy to combine our expertise with yours!
By considering TURBOARD as an OEM partner for BI integration into your software, you will earn advantages other alternatives cannot provide, as outlined below:
| With Other BI Vendors | By Partnering with TURBOARD | |
|---|---|---|
| Pricing |
|
|
| Time to Market |
|
|
| Flexibility & Extensibility |
|
|
| Support |
|
|
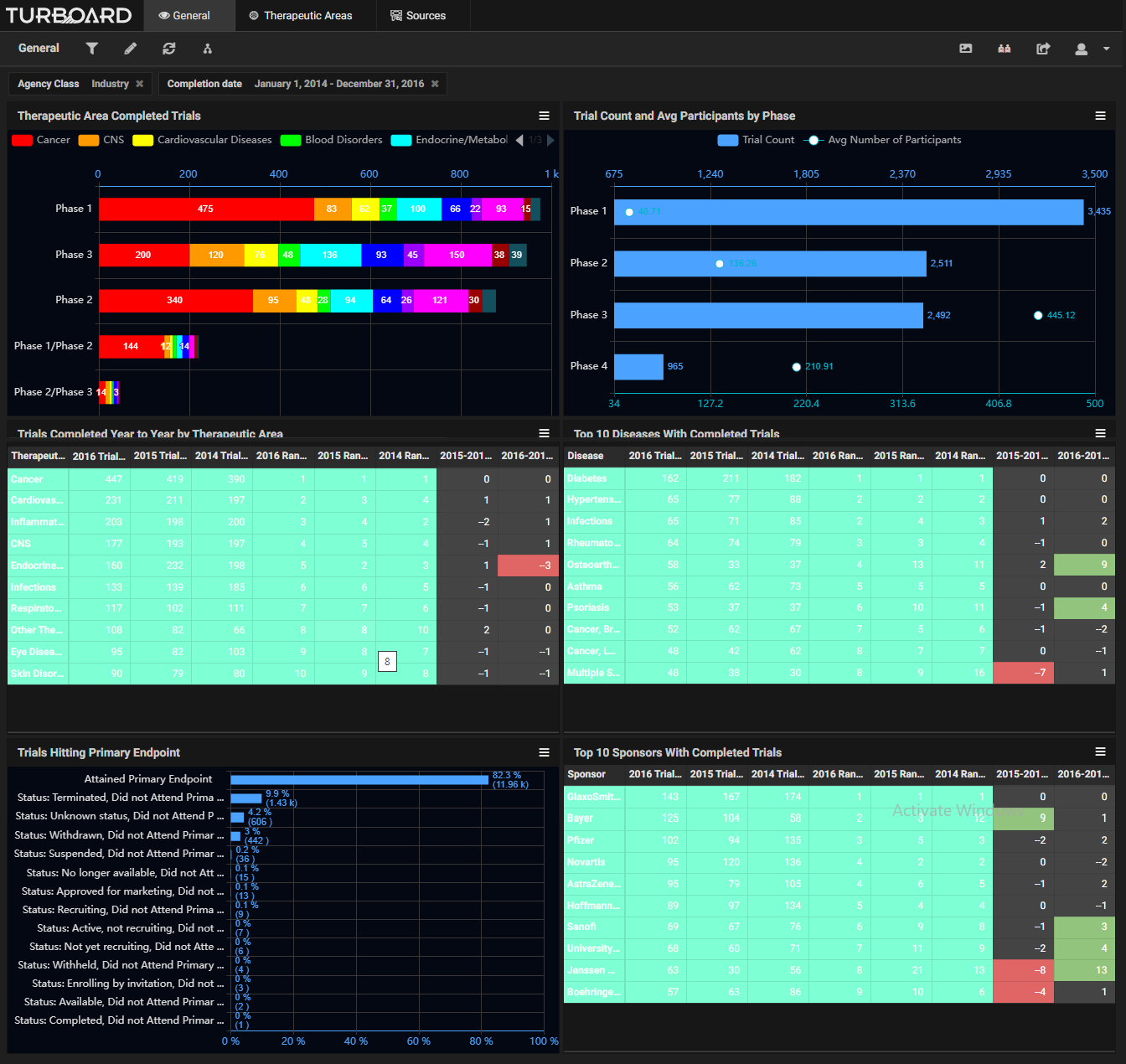
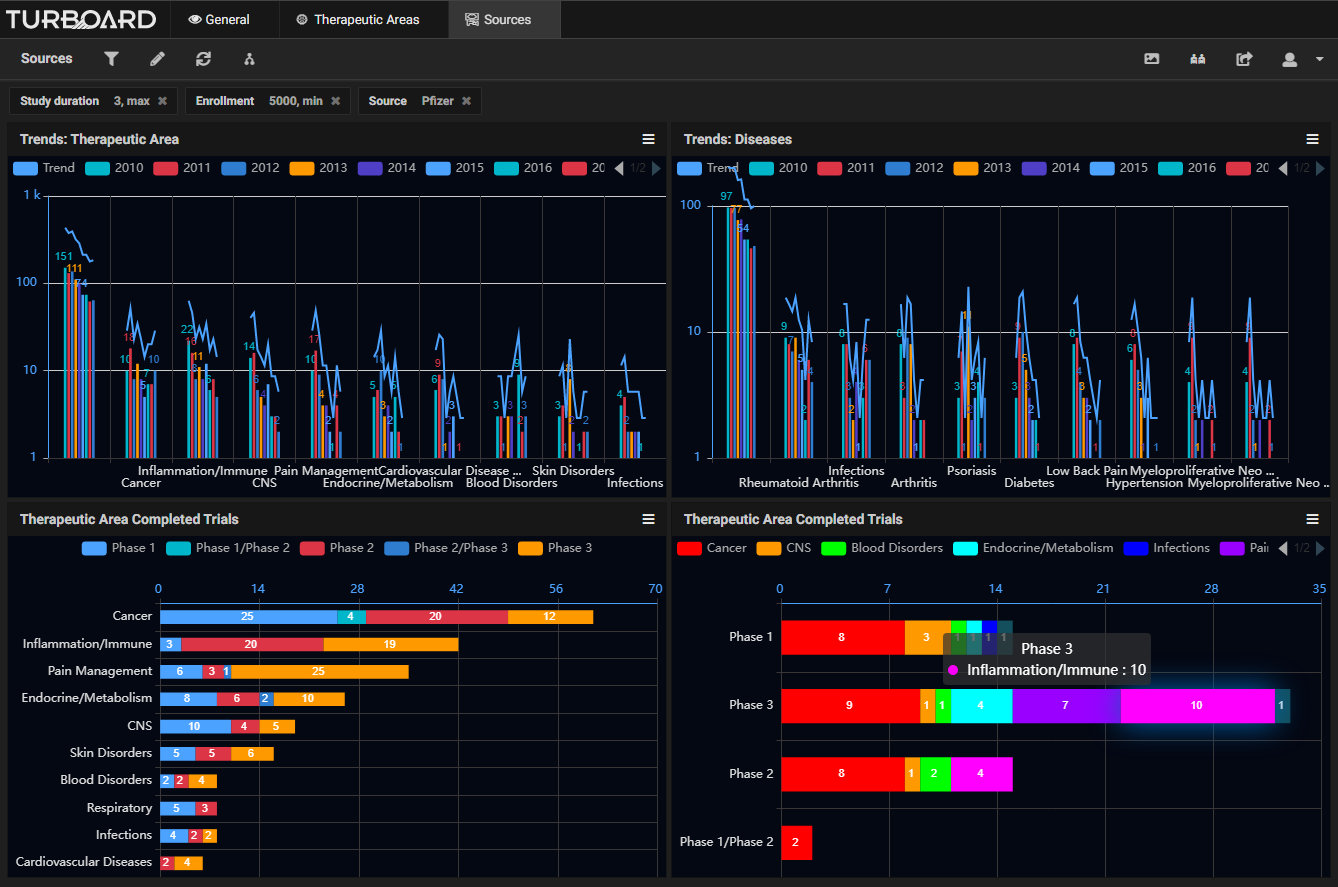
Sample Showcases Demonstrating TURBOARD’s Analytic Capabilities in Clinical Trials Data
The data is automatically collected and made available to users from various up-to-date, open source clinical databases. These reports and analytics can be viewed according to various dimensions, such as study duration, purpose, type, masking type, trend, diseases, sources/sponsors, therapeutic areas, trials count, success status and rates, and more, with drilling-down and slicing options. By monitoring these analyses, a sponsor can adjust a trial development plan, compare the performances of sponsors, and decide whether to opt for a certain clinical trial or apply a fail-fast policy to eliminate it. The sponsor will earn insights into:
|
|
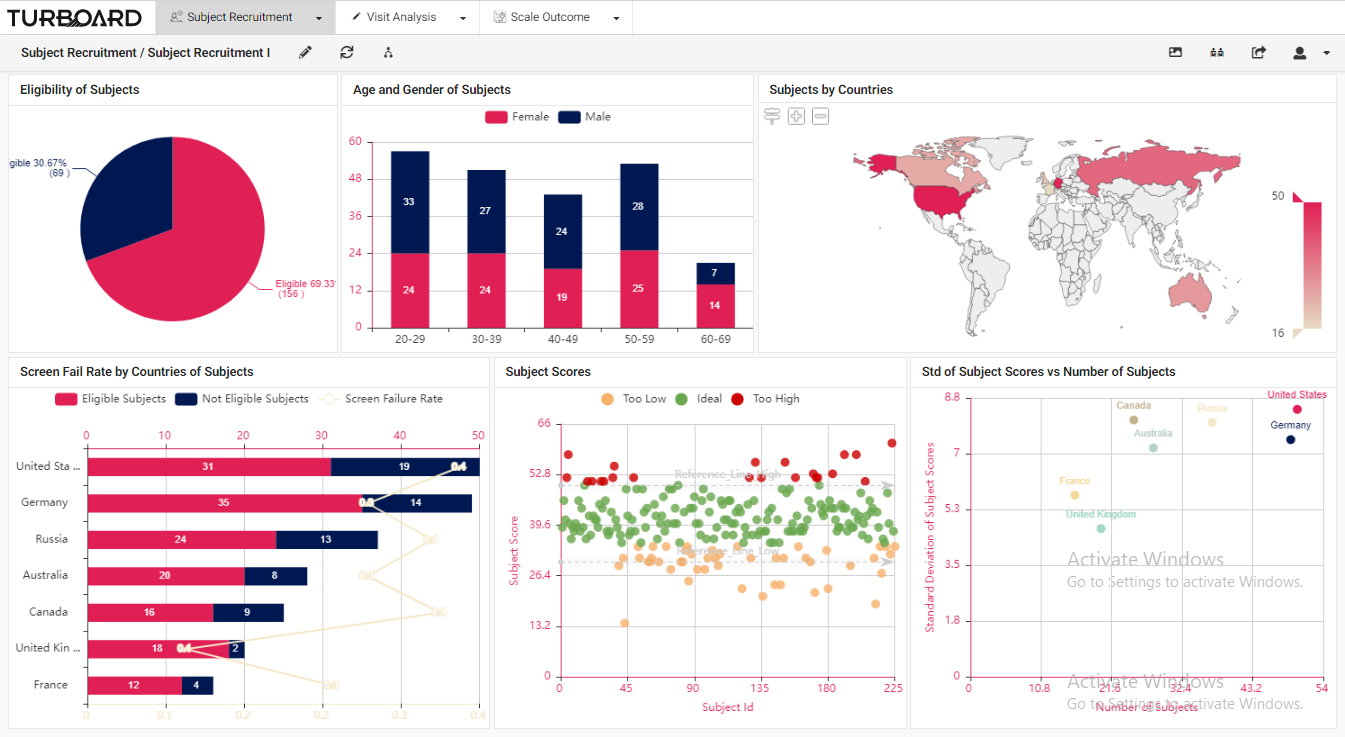
Trial sponsors and CROs can monitor, follow up, and prepare any kind of report for their ongoing clinical trial(s) via a perceptive platform. For a selected clinical trial, users can monitor participants’ recruitment data, eligibility, visit/lab/test results, and rating scales’ details. 1. Subject Recruitment: Trial participants’ reports and analyses with multi-filters and slicing options.
|
|
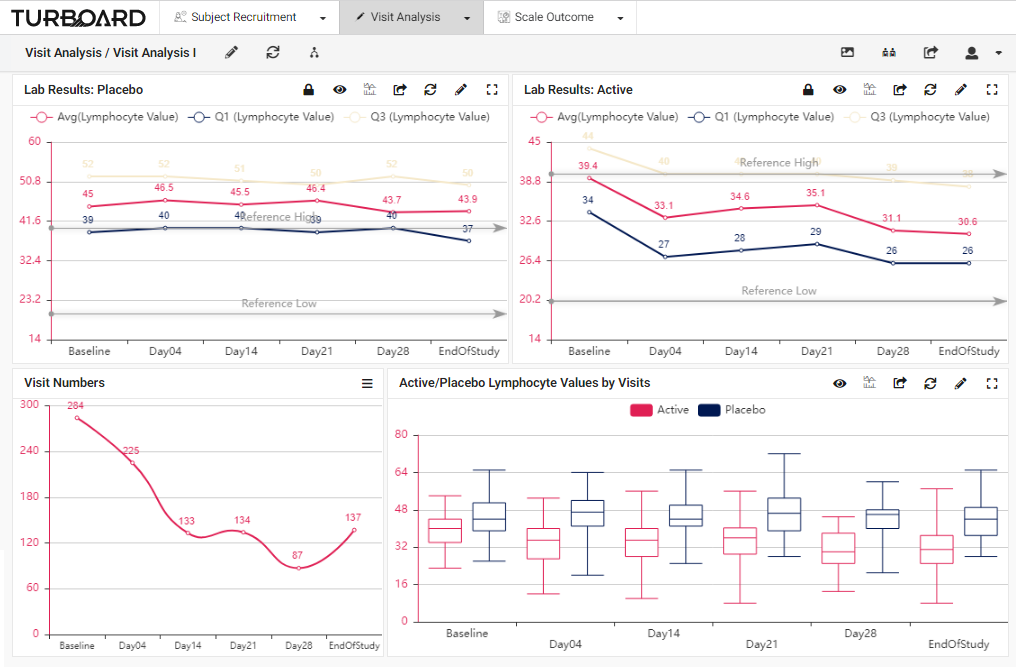
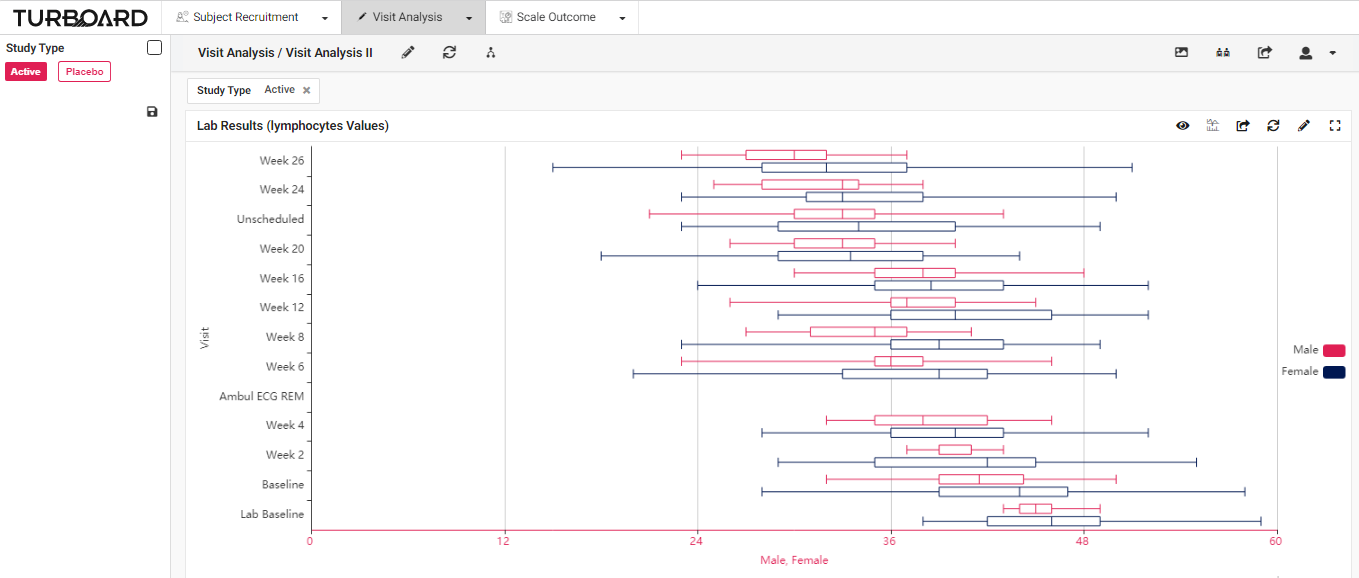
2. Visit Analysis: Visualized reports and analysis of lab results, values, and visits with multi-filters and slicing options.
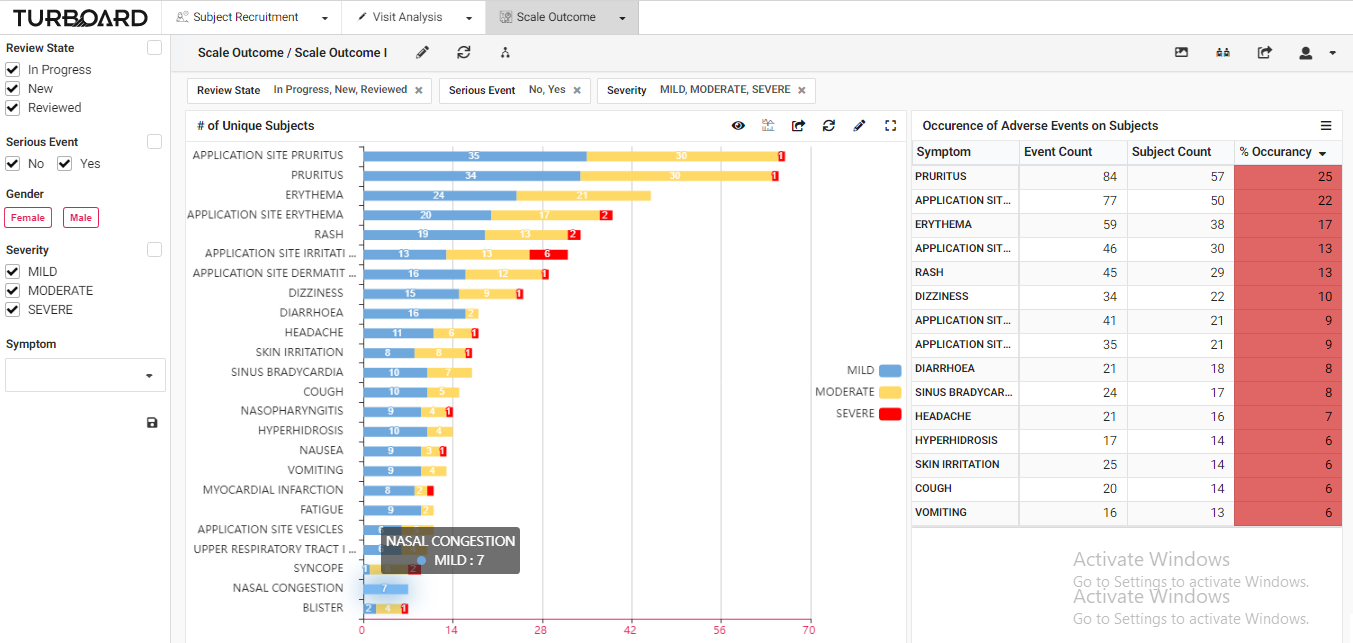
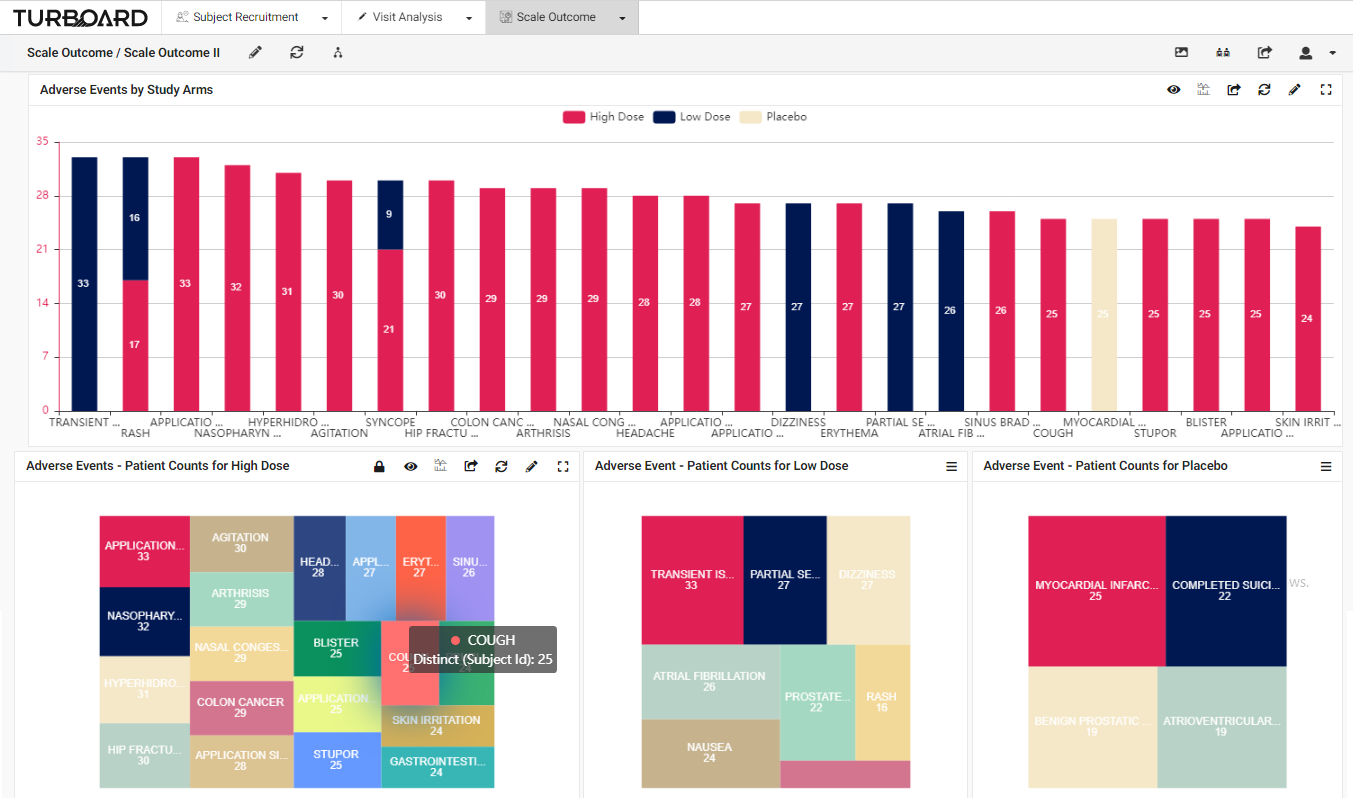
3. Scale Outcome: Visualized reports and analysis of rating scale outcome with multi-filters and slicing options.
To reveal striking insights hidden in your own data, discover